Cis but 2 Ene
Butene auch Butylene sind eine Gruppe von vier isomeren Kohlenwasserstoffen mit der allgemeinen Summenformel C 4 H 8 die über eine CC-Doppelbindung verfügen. TiGER is a database developed by the Bioinformatics Lab at Wilmer Eye Institute of Johns Hopkins University.

Among Cis But 2 Ene Trans But 2 Ene Which One Is Polar Why Youtube
Trans-but-2-ene is also known as E.
. Doc Browns Chemistry Advanced Level Pre-University Chemistry Revision Notes for UK IB KS5 AAS GCE advanced A level organic chemistry students US K12 grade 11 grade 12 organic chemistry courses on molecular spectroscopy analysing H-1 NMR spectra of E-but-2-ene Z-but-2-ene. Solution for H2Ni cis but-2-ene. Sides of a double bond.
The H-1 hydrogen-1 proton NMR spectrum of E-but-2-ene and Z-but-2-ene. The Zaitsev Rule favors formation of 2-butene cis trans over 1-butene. Position isomerism also called regioisomerism - constitutional isomers in which a functional group or substituent changes.
Cis-but-2-ene is also known as Z-but-2-ene. The thiol-ene reaction also alkene hydrothiolation is an organic reaction between a thiol and an alkene to form a thioetherThis reaction was first reported in 1905 but it gained prominence in the late 1990s and early 2000s for its feasibility and wide range of applications. B number from the end that gives the first carbon of the CC the lowest number.
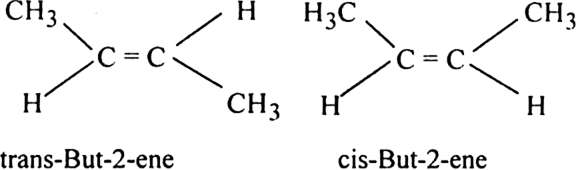
The two methyl groups may be directed on the same side of the double bond cis-or Z or they may be directed away from one another trans- or E. As per EPC diagram the mixed state has higher potential energy that is increasing temperature wou. Think about this relatively uncomplicated molecule.
Then the two identical methyl groups are either cis or trans to each other and the two identical hydrogen atoms are either cis or trans to each other. This reaction is accepted as a click chemistry reaction given the reactions high yield stereoselectivity high. The PEC diagram for the dissolution of the mineral chalcocite Cu2S in water is shown to the right.
The ambiguity comes from the definition of similar groups. The cis-trans definition is unambiguous only when you have two different groups on one of the alkene carbons and the same two groups on the other carbon as in but-2-ene. Look for example at the 12-dichloroethene and but-2-ene cases.
These differences can be very small as in the case of the boiling point of straight-chain alkenes such as pent-2-ene which is 37 C in the cis isomer and 36 C in the trans isomer. ヘキセンHexeneはC 6 H 12 という分子式を持つアルケンである hexという接頭辞は分子に6つの炭素原子があることを意味し-eneという接尾辞は2つの炭素原子が二重結合で結ばれるアルケンであることを意味している 鎖の中の二重結合の配置と幾何によりいくつかの異性体が存在. But it doesnt always work.
Can you easily translate cis- and trans- into Z- and E-. 7023 7010 7035 2731 7249 9832 7544 7545 1585 SUBSTANCE DEA NUMBER CSA SCH NARC OTHER NAMES AB-FUBINACA N-1-amino-3-methyl-1-oxobutan-2-yl-. This is clearly a cis- isomer.
The terms cis- and trans- are still used but this usage is being phased out. CH 3 2 CCHCHClCH 3 is 4-chloro-2-methyl-2-pentene. The cis isomer of pent-2-ene has a boiling point of 37 o C but the boiling point of the trans isomer is 36 o The difference is small because the bond polarity is low.
If you are. Sie zählen damit zu den AlkenenZwei der Isomere unterscheiden sich durch cis-trans-Isomerie. Alkynes - compounds containing carbon-carbon triple bonds.
This is common for the carbon-carbon double and triple bonds which have the respective suffixes ene and yne. This is most commonly seen when the skeleton or backbone consists of a carbon chain. In fact the situation is even more complicated than it looks because but-2-ene exhibits geometric isomerism.
You get a mixture of two isomers formed - cis-but-2-ene and trans-but-2-ene. CH2CH3 cis-1-ethyl-2-methylcyclobutane trans-1-methyl-3-propyl-cyclohexane CH3 CH2CH2CH3 CHCH2CH2CH3 CH3 2-cyclobutylpentane or 1-methylbutylcyclobutane 3. ALKENES a use the longest chain containing the CC and replace -ane with -ene.
You might think that for simple cases cis- will just convert into Z- and trans- into E-. Butene sind unter Standardbedingungen farblose brennbare Gase mit einer größeren Dichte als Luft. The database contains tissue-specific gene expression profiles or expressed sequence tag EST data cis-regulatory module CRM data and combinatorial gene regulation data.
The differences between cis and trans isomers can be larger if polar bonds are present as in the 12-dichloroethenesThe cis isomer in this case has a boiling point of 603 C while the trans. Due to the polar nature of the bonds in 12-dichloroethylene the boiling point of the cis isomer is 603 o C whereas that of the trans isomer is 475 o C the C-Cl dipole moments. Tissue-specific Gene Expression and Regulation TiGER.
Skeletal isomerism also called chain isomerism - structural isomers in which components of the skeleton are arranged in a different order. The products are but-1-ene CH 2 CHCH 2 CH 3 and but-2-ene CH 3 CHCHCH 3. Cis-2-Butene C4H8 CID 5287573 - structure chemical names physical and chemical properties classification patents literature biological activities safety.
Halogens on the other hand do not have a suffix and are named as substituents for example.
What Is The Symmetry In Cis 2 Butene Quora

5 2 Geometric Isomers And E Z Naming System Chemistry Libretexts

Get Answer Draw The Cis And Trans Isomers Of 2 Butene Otosection
Out Of The Two Trans But 2 Ene And Cis But 1 Ene Which Is More Stable And Why Wired Faculty

File Cis 2 Butene Svg Wikipedia

0 Response to "Cis but 2 Ene"
Post a Comment